Name the Largest Family of Cell Surface Receptor
Image: "Solid cell nests" by
Ken Berean. License: CC BY-SA two.0
Receptors
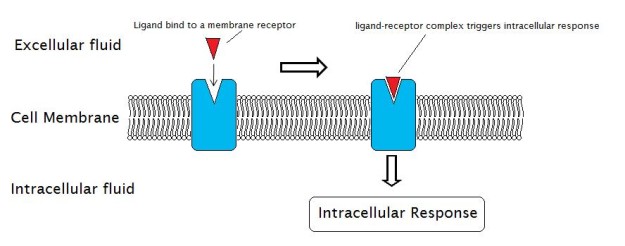
Prototype: "External Reactions and the Internal Reactions of Receptors" by Laozhengzz. License: Public Domain
A receptor is a molecule that receives signals (chemical or hormonal) from outside the cell and is usually located on the cell surface. Receptors are proteins that undergo a conformational change upon attachment of their corresponding signaling molecule, which in turn induces a chain reaction (as well known as signal transduction) inside the cell leading to various cellular responses, including cell death.
The signaling molecule that binds to the receptor (also known as a ligand) can exist a peptide, a hormone, neurotransmitter, drug, toxin, etc. Each receptor possesses 2 functional domains: the recognition domain which binds ligands and the coupling domain which is involved in signal transduction.
Cell Surface Receptors
Jail cell surface receptors are transmembrane proteins embedded into the plasma membrane which play an essential part in maintaining communication between the internal processes within the cell and various types of extracellular signals.
Such extracellular signals include hormones, cytokines, growth factors, neurotransmitters, lipophilic signaling molecules such equally prostaglandins, and cell recognition molecules. When any of these ligands bind to their corresponding receptor, a conformational change is triggered which initiates an intracellular signaling pathway. Annotation that each ligand has its own specific cell surface receptor.
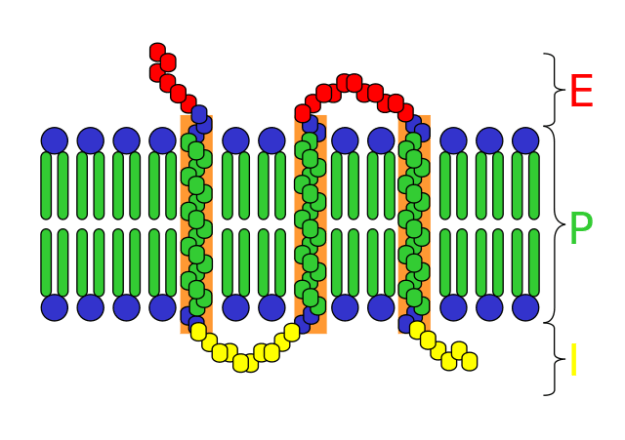
Image: "A schematic of a transmembrane receptor; E = extracellular infinite P = plasma membrane I = intracellular space" past Mouagip. License: CC By-SA 3.0
Additionally, cell surface receptors are specific to individual jail cell types and thus are also known as cell-specific proteins. These receptors regulate a multitude of biological pathways required for prison cell growth, survival, differentiation, proliferation, as well as many other cellular processes. Cell surface receptors are responsible for nearly of the signaling in multicellular organisms.
Cell surface receptors take the following components/domains:
- The extracellular domain which binds ligands and is exposed to the outer surface of the cell; also known every bit the recognition domain
- The membrane-spanning region made up of hydrophobic poly peptide molecules
- The intracellular domain which is in contact with the cytoplasm; as well known as the coupling domain
Several factors govern the properties of these domains, including the size and extent of the domains, which may vary according to the type of cell surface receptor.
Cell Surface Receptors: Types
Cell surface receptors are generally classified into the following categories:
- Ligand-gated ion aqueduct-linked receptors
- Enzyme-linked receptors
- K-protein-linked receptors
Ion channel-linked receptors
Ion channel-linked receptors also referred to as ionotropic receptors, are responsible for regulating the transduction of chemic signals across the cell membrane in response to the chemical messenger (e.m., neurotransmitter) binding. This course of receptor regulates the opening or endmost of ion channels that permit ions like Na + , K + , Ca 2+ , or Cl − , etc. to motility across the plasma membrane.
Ion channels are pore-forming proteins also referred to as jail cell-membrane bound receptors. Ions pass downwardly their electrochemical slope through ion channels without requiring ATP or metabolic energy. Ion channels are mostly found on synaptic structures which are primarily (just non exclusively) involved in neuronal activities. Ion channels are, therefore, an important component of the nervous system because they mediate conduction beyond nervus synapses when activated by neurotransmitters.
Ion channels too play a vital role in exerting cellular response to toxins and venoms. Various biological processes involving fast changes in cells such as the contraction of cardiac , skeletal , and smooth muscles , activation of T-cells, and the release of hormones are also mediated through ion channels.
Downstream mechanism
When a ligand binds to ion-channel linked receptors, the extracellular domain of the receptor undergoes changes in its conformation, opening a aqueduct across the plasma membrane. This allows specific ions (such as Na + , Ca 2+ , H + , and Mg two+ , etc.) or other cardinal molecules to laissez passer through the open channel. The membrane-spanning region of these receptors helps to form a channel through which ions tin can pass.
Ligands that demark to ion channel-linked receptors include neurotransmitters and peptide hormones and the passing molecules are ions such equally sodium (Na + ) and potassium (Chiliad + ).
The amino acids that occupy the membrane-spanning region of ion-channel receptors are hydrophobic in nature, making information technology easier for the membrane phospholipid fat acid bondage to collaborate with them. On the other hand, amino acids lining the inside of these channels are hydrophilic, assuasive easy passage of ions and water. The ion channels (or pores) remain open only for a limited time, after which, the ligand dissociates from the receptor making it available to bind with a new ligand.
Non-chemic stimuli can also cause the ion aqueduct receptors to act in the same style. Such non-chemic stimuli include changes in electric accuse or mechanical disturbances of the membrane.
Enzyme-linked receptors
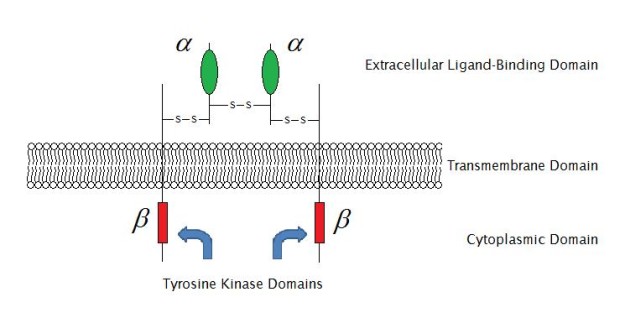
Paradigm: "Enzyme-linked receptor structure (construction of IGF-1R) " past Laozhengzz. License: Public Domain
Enzyme-linked receptors are typically single-pass transmembrane proteins that act as enzymes or are associated with enzymes. Enzyme-linked receptors accept both an extracellular binding site for chemic signaling and an intracellular domain whose catalytic action is controlled by the binding of an extracellular ligand and are thus likewise called catalytic receptors. There are half dozen types of enzyme-linked receptors:
- Receptor tyrosine kinases which phosphorylate specific tyrosine residues on specific intracellular signaling proteins (EGFR); they bind to polypeptide growth factors which are responsible for controlling jail cell proliferation and differentiation
- Tyrosine-kinase-associated receptors which are enzymes that associate with intracellular proteins that take tyrosine kinase activity (Cytokines)
- Receptor-similar tyrosine phosphatases which remove phosphate groups from tyrosines of their target intracellular proteins
- Receptor serine/threonine kinases which phosphorylate specific serines or threonines on associated gene regulatory proteins
- Receptor guanylyl cyclases which direct catalyze the production of cyclic GMP in the cytosol (natriuretic peptide receptor)
- Histidine-kinase-associated receptors which activate a two-component signaling pathway where the kinase phosphorylates itself on histidine residues (autophosphorylation) then immediately transfers the phosphate to a second intracellular protein; not present in animal cells
Downstream mechanism
When the enzyme-linked receptor or an enzyme associated with this type of receptor is activated, a multitude of intracellular pathways are finer regulated. The intracellular domains of enzyme-linked receptors are associated with an enzyme, direct interact with an enzyme, or itself is the enzyme.
Irrespective of large intracellular and extracellular domains of enzyme-linked receptors, a single blastoff-helical region of the peptide chain is responsible for forming the membrane-spanning region of enzyme-linked receptors. Activation of the enzyme takes place as a result of the point transmitted through the aqueduct after a ligand binds to the extracellular domain of the receptor.
In most cases, activation of the enzyme takes place because the receptor is dimerized upon binding of a ligand. The activated enzyme leads to an intracellular cascade of events executing the response.
Chiliad-protein linked receptors
Thousand-protein-coupled receptors (GPCRs) are the largest prison cell surface receptors, composed of 7 transmembrane proteins in the plasma membrane. GPCRs are responsible for activating the trimeric membrane-bound G-proteins (GTP bounden proteins) which subsequently activate either an ion channel (effector) or an enzyme in the jail cell membrane.
G-proteins proteins function every bit an intermediate transducer molecule that plays a vital part in regulating intracellular functions through a secondary mechanism which is in turn activated past G-poly peptide coupled receptors. Many different types of K-protein-coupled receptors are known, such as the acetylcholine (Ach) receptor, β-adrenergic receptor, metabotropic glutamate receptors, sure olfactory receptors, receptors for peptide hormones, and rhodopsin (a photosensitive receptor).
Many neurotransmitters, neuropeptides, peptide hormones, as well as a number of others tin actuate G-poly peptide coupled receptors. GPCRs are responsible for targeting various signaling pathways, including sensory perception such as sight, taste, odour , and pain sensations. GPCRs are among the nigh important cell surface receptors, with virtually half of the drugs nosotros utilize exerting their activeness by modifying these receptors.
Downstream mechanism
When the alpha subunit is reversibly bound to Gross domestic product, GPCR is inactivated. However, the GDP tin exist exchanged for GTP with the help of a guanine-nucleotide substitution gene (Global environment facility), allowing for receptor activation.
Later a ligand binds to GPCR, the G-protein is activated. The activated G-protein, in plow, activates either an ion channel (effector) or an enzyme in the jail cell membrane. A circadian series of events takes place as a effect of jail cell signaling with G-protein-linked receptors.
Source: https://www.lecturio.com/magazine/cell-surface-receptors-types-downstream-mechanisms/
Postar um comentário for "Name the Largest Family of Cell Surface Receptor"